
Introduction: From DNA Chains to Life Chains
Barely seven decades ago, in 1953, James Watson and Francis Crick unveiled the double-helix structure of DNA. Their discovery revealed DNA as a “chain” of nucleotides encoding life’s blueprint—often called the “molecule of heredity.” Composed of four chemical bases (adenine, thymine, cytosine, guanine), DNA strands pair up in a twisted ladder formation (the iconic double helix). This elegant chain of genetic code directs the development and functioning of all living organisms. Over the following decades, scientists deciphered how sequences of DNA bases form genes, which in turn produce proteins that carry out life’s processes.
From DNA to Life: With DNA’s structure and role understood, biology entered an era of “reading” the genetic code (gene sequencing) and eventually “writing” or editing it. The term “life chains” symbolizes how humanity learned to manipulate DNA chains to alter living organisms. In the 1970s, pioneers like Paul Berg, Stanley Cohen, and Herbert Boyer developed techniques to cut and splice DNA, creating the first recombinant DNA molecules. This was the birth of genetic engineering—scientists could now rearrange DNA chains and thereby modify the traits of life forms. Over time, these advances gave rise to genetically modified organisms (GMOs), gene therapies, and even attempts to create synthetic life.
Today, we stand at the threshold of a genetic frontier. Tools like CRISPR allow us to edit genes with unprecedented precision, effectively relinking DNA chains to forge new outcomes in organisms. We are moving from simply observing DNA sequences to actively rebuilding life’s code—transforming “DNA chains” into “life chains” that span species and ecosystems. This e-book explores that trajectory: from the first GMOs to gene-edited mosquitoes and CRISPR cures, and onward to the ethical and regulatory chains we must forge to guide this fast-evolving genetic world.
Genetically Modified Products: History, Modern Varieties, and Global Records
Genetically Modified Organisms (GMOs) are living things whose DNA has been artificially altered through genetic engineering. This section traces the history of GMO development, outlines modern GMO varieties in agriculture and other fields, and presents global records and facts about GMO adoption around the world.
Early History of Genetic Modification
Long before modern GMOs, humans had been indirectly modifying organisms through selective breeding and hybridization for thousands of years. However, the modern era of genetic modification began in the 1970s:
- 1973 – First DNA Recombinant: Researchers Cohen and Boyer combined DNA from different organisms, creating the first recombinant bacteria. This landmark experiment demonstrated that genes could be cut from one species and pasted into another, forming the basis of GMO technology.
- 1982 – First GMO Product (Medical): By the early 1980s, this technology yielded its first commercial success in medicine. In 1982, the FDA approved biosynthetic human insulin produced by genetically engineered bacteria. Branded as Humulin, this insulin was identical to human insulin and replaced insulin derived from pigs and cows. It became the first genetically modified product available to consumers, heralding biotechnology’s medical potential.
- 1980s – Agricultural Experiments: In 1983, scientists created the first genetically modified plant (an antibiotic-resistant tobacco), proving that foreign genes could be introduced into plant genomes. This paved the way for GMO crops.
- 1994 – First GM Food Crop: The Flavr Savr tomato became the first genetically engineered food to receive FDA approval for sale in the U.S.. It was modified to delay ripening and prevent softening, theoretically allowing vine-ripe flavor with longer shelf life. While Flavr Savr tomatoes reached supermarket shelves in 1994 amid much public interest, they were not a commercial success and were withdrawn a few years later. Nonetheless, this opened the door for other GMO foods.
Rise of GMO Crops and Modern Varieties
After 1994, a wave of genetically modified crops were developed, especially in staple commodities:
- Insect-Resistant Crops: Scientists inserted a gene from the soil bacterium Bacillus thuringiensis (Bt) into plants like corn and cotton, enabling the plants to produce a protein toxic to certain pests. By the late 1990s, Bt corn and Bt cotton were widely adopted by farmers. Bt crops have been shown to reduce the need for external insecticide spraying, since the plant itself provides protection. For example, Bt cotton usage grew rapidly in the U.S., China, India and other countries, dramatically cutting losses from bollworm pests.
- Herbicide-Tolerant Crops: Another popular modification made crops resistant to a specific herbicide. The most famous are “Roundup Ready” soybeans, corn, and canola, engineered to tolerate glyphosate herbicide. First introduced in 1996, these allow farmers to spray glyphosate to kill weeds without harming the crop. This trait also saw huge adoption; by the 2010s, over 90% of soybeans grown in the U.S. were genetically modified for herbicide tolerance.
- Virus-Resistant and Other Traits: Papaya ringspot virus once threatened to wipe out Hawaii’s papaya industry. In 1998, researchers deployed a virus-resistant GMO papaya (Rainbow papaya), inserting a bit of viral DNA into the papaya genome to vaccinate the plant. This saved the papaya industry and is a celebrated case of GMO use. Other trait innovations include potatoes that resist bruising and browning, “Golden Rice” enriched with vitamin A precursor (to combat malnutrition), and biofortified cassava and bananas. By 2015, “Arctic apples” that don’t turn brown when cut, and “Innate potatoes” with reduced acrylamide and browning, had also been approved.
- GM Animals: Although less common than plants, a few genetically modified animals have been developed. In 2015, after two decades of review, the FDA approved the AquaAdvantage salmon, a fast-growing Atlantic salmon that contains a gene from Chinook salmon (allowing it to reach market size quicker). More recently in 2020, the FDA approved a genetically modified pig (GalSafe pig) for both food and medical use. This pig is engineered to lack a sugar on its cells that triggers meat allergies in some people; it may also provide safer organs/tissues for transplantation into humans.
Global Adoption and Records
Since 1996, when GMO crop commercialization began, global adoption has surged. Farmers have found many GM varieties economically attractive due to improved pest control and yield stability. Some key global records and statistics include:
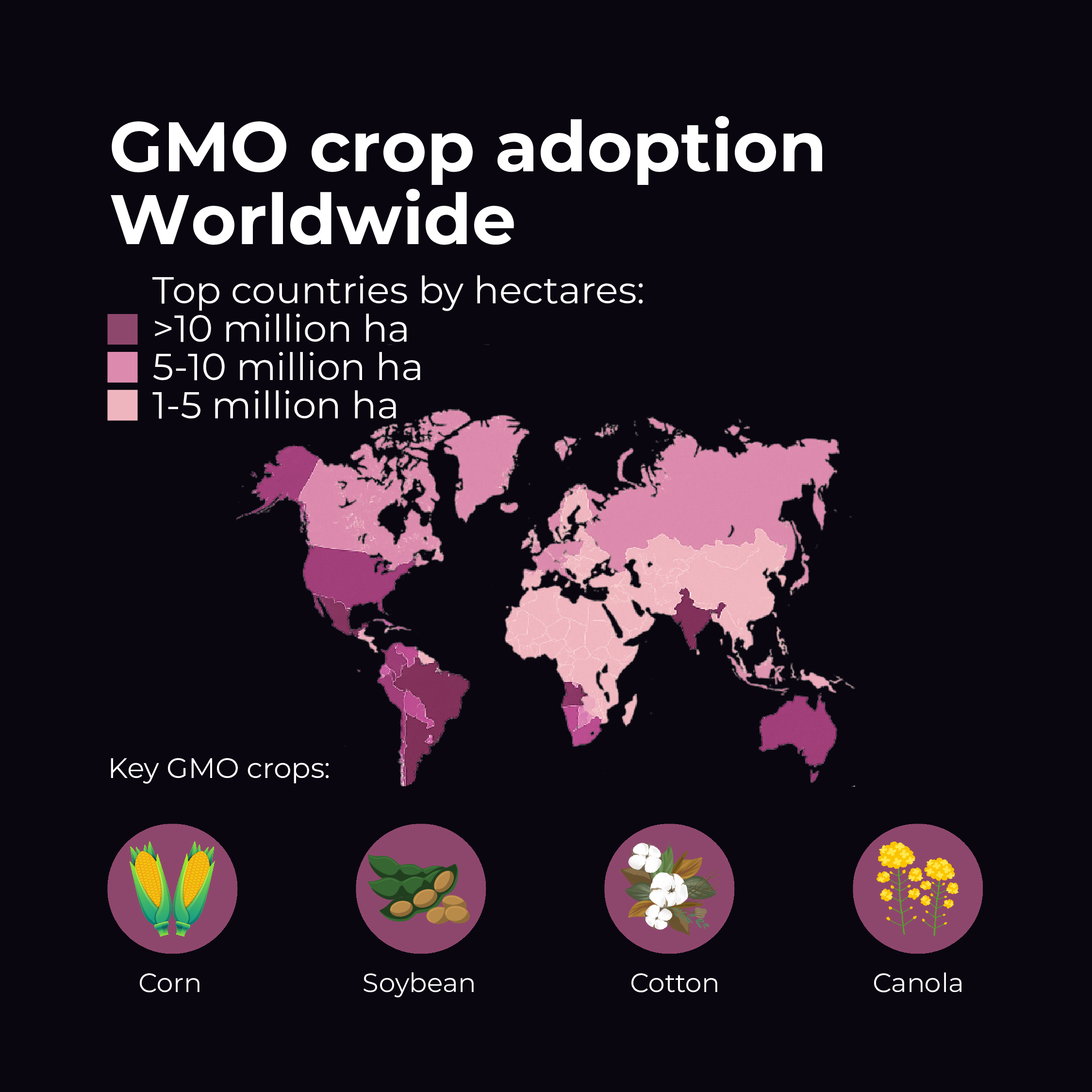
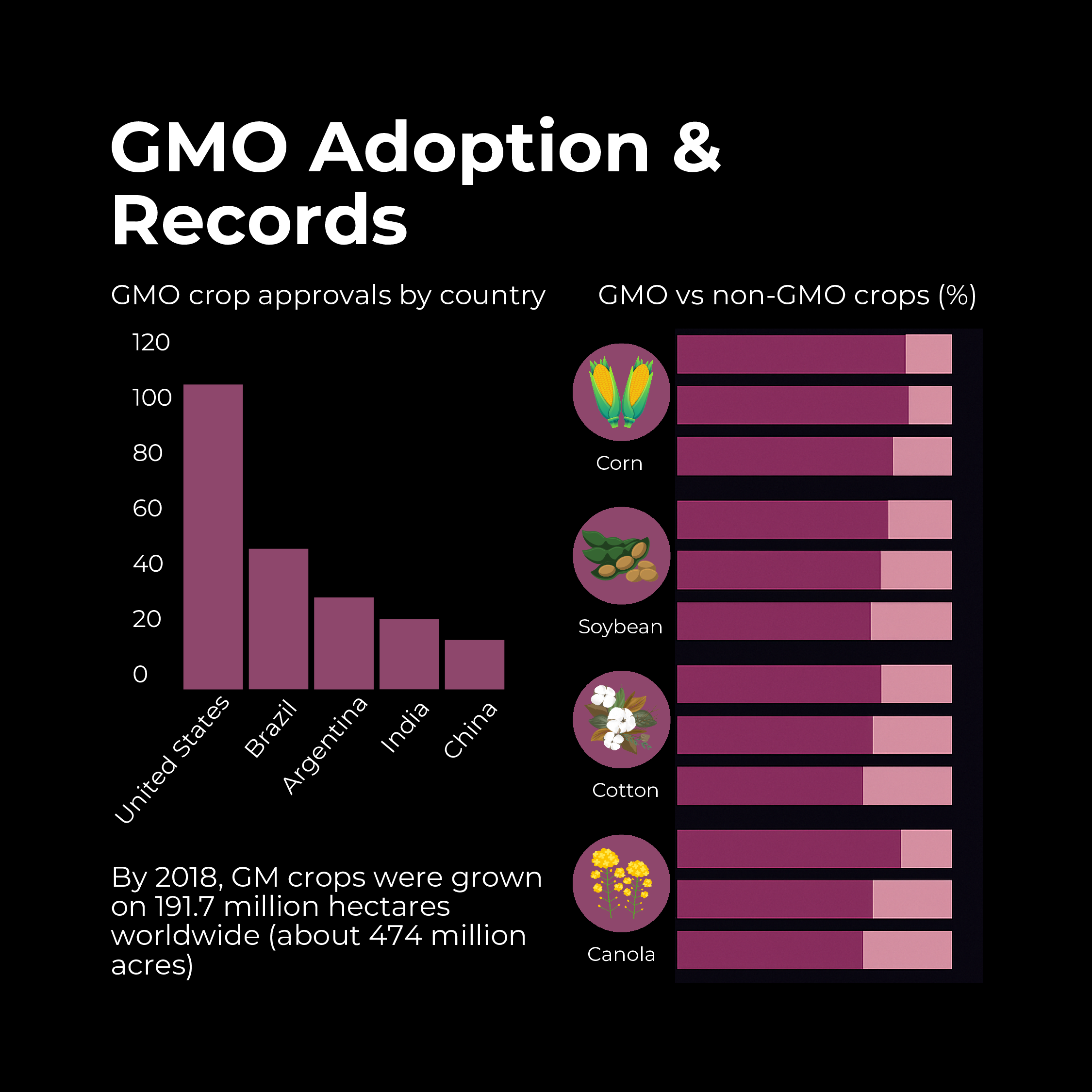
- Rapid Adoption: GMO crops have been the fastest-adopted agricultural technology in modern history. By 2018, GM crops were grown on 191.7 million hectares worldwide (about 474 million acres). This represents an 113-fold increase in acreage from 1996 to 2018 – a stunning growth rate. The U.S., Brazil, Argentina, Canada, and India are leading adopters, planting the majority of those hectares.
- Leading Crops: The main GM crops by area are soybeans, corn (maize), cotton, and canola. For instance, around 95% of soybeans in the U.S. are GM, as are ~90% of corn and cotton. Other GM crops with significant acreage include sugar beet, alfalfa, and papaya (in Hawaii). To date, more than 70 different GM crop varieties have been approved in one or more countries, though only a subset reach large-scale cultivation.
- Top Countries: The United States leads in biotech crop area (~75 million hectares in 2018), followed by Brazil (~50 million), Argentina, Canada, and India. Notably, developing countries now plant over half of global GMO crop area, reflecting adoption in Asia, Latin America, and Africa. For example, India has embraced Bt cotton on virtually all its cotton acres, dramatically reducing pesticide poisonings among farmers and boosting yields.
- Production Records: GMOs have enabled record harvests in some cases. The use of insect-resistant and herbicide-tolerant crops contributed to consistent year-on-year yield increases in crops like corn and soy during the 2000s and 2010s. By reducing losses to pests and weeds, GM technology has been credited with improving farm productivity and securing the food supply. For instance, Bt corn adoption correlated with reductions in European corn borer pest damage and higher average corn yields in the U.S.
- Economic and Environmental Impact: It’s estimated that from 1996 to 2016, biotech crops contributed to $186.1 billion USD in economic gains for farmers (mostly in developing countries) and reduced pesticide spraying by 619 million kg (a 8.1% reduction). They also helped decrease agriculture’s carbon footprint by enabling low-till farming (which uses less fuel and preserves soil carbon).
Despite these successes, GMO adoption is uneven globally. Many European nations have been cautious; the European Union has strict regulations that have limited cultivation (only one GM maize is grown in EU, mostly in Spain). However, even in Europe, millions of tons of GM soybean and corn are imported yearly for animal feed. Meanwhile, China is investing in GM technology (e.g. approving GM papaya and cotton, and recently GMO corn/rice field trials) but has yet to fully commercialize staple GM food crops domestically.
Modern GMO Innovations
The scope of genetic modification continues to expand beyond the early generation of crops:
- Genome Editing in Agriculture: Newer genome-editing tools (like CRISPR, covered later) are enabling “GMOs 2.0” – precise edits without foreign genes. By 2019, the first CRISPR-edited agricultural products emerged. For example, U.S. companies developed a gene-edited soybean with high oleic oil (for heart-healthy cooking oil). Japan has released a CRISPR-edited tomato rich in GABA (a beneficial neurochemical) in 2021. Regulators are grappling with how to classify these, but they promise traits like drought tolerance, nutritional enhancement, and disease resistance through small DNA tweaks rather than transgenes.
- Record Approvals: By 2020, the U.S. FDA and USDA had approved a wider array of GM foods than ever. In one notable case, pink-fleshed pineapples engineered to produce lycopene (an antioxidant pigment) were approved by the FDA in 2016. A fast-growing GM salmon (AquaAdvantage) finally reached U.S. markets in 2021, becoming the first genetically altered animal food to be sold. The FDA also completed its first consultation on a CRISPR-edited plant (soybean) in 2019, signaling that gene-edited crops are joining the ranks of “genetically modified products,” even if regulators classify them separately.
- Global Records: The most recent figures (as of 2018) indicated 26 countries were planting biotech crops, and another 44 countries imported or used them, meaning a total of 70 countries were benefiting from GMOs either through cultivation or consumption. Over that period, around 17 million farmers (the majority smallholder farmers in developing countries) adopted GM seeds – a record participation for any agricultural innovation.
Going forward, GM products are diversifying. We see genetically modified microbes producing biofuels and green chemicals, gene-edited livestock with disease resistance (e.g. pigs resistant to PRRS virus), and efforts to use GM plants to produce pharmaceuticals (plant-made vaccines). The concept of “Life Chains” is exemplified by these developments – DNA chains are being rearranged across the tree of life to forge new beneficial links, from farm fields to pharmacy shelves.
Genetic Fingerprinting in the Insect World: Combating Mosquitoes from DDT to GMOs
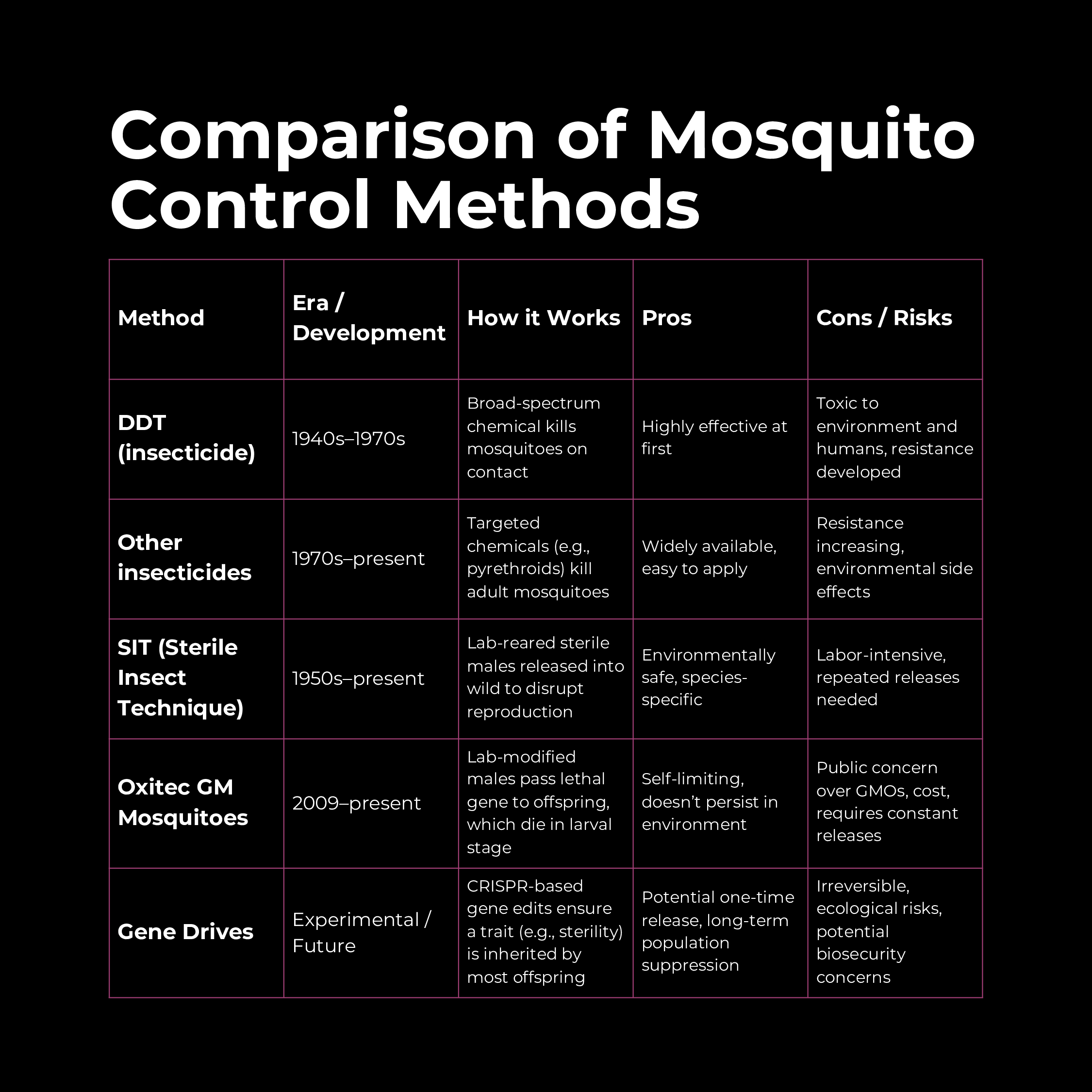
Mosquitoes have long been humanity’s deadliest adversary, spreading diseases like malaria, dengue, Zika, and yellow fever. Over history, our methods of fighting mosquitoes have evolved from chemical to genetic. This section explores the journey from DDT (a potent chemical insecticide) to genetically modified mosquitoes, including the example of Oxitec’s GM mosquitoes, and examines future bio-techniques and bio-threats involving insects.
The DDT Era: Chemical Warfare on Mosquitoes
In the mid-20th century, the world turned to chemicals to wage war on mosquitoes. DDT (dichloro-diphenyl-trichloroethane) was introduced in the 1940s as the first modern synthetic insecticide. It proved spectacularly effective:
- Allied forces in World War II used DDT to suppress malaria and typhus among troops. Post-war, DDT was deployed globally in public health campaigns; spraying homes and wetlands in malaria-endemic regions led to steep drops in mosquito populations and malaria cases.
- By the 1960s, DDT helped eradicate malaria from several countries and dramatically reduced it in others. It was cheap, persistent, and deadly to insects – an apparent miracle chemical.
However, DDT’s very persistence became its downfall. In 1962, Rachel Carson’s Silent Spring sounded the alarm on DDT’s ecological impacts. Scientists observed that DDT accumulated in food chains, harming wildlife (famously thinning eggshells of eagles and falcons) and potentially posing human health risks. Many insect species also evolved resistance to DDT due to heavy usage.
As evidence of environmental damage mounted, the U.S. banned DDT in 1972, and most other countries restricted its use. By 2004, the global Stockholm Convention on Persistent Organic Pollutants banned DDT worldwide except for limited malaria control where no alternatives exist. Today, DDT is still sparingly used indoors in some African and South Asian countries to combat malaria, under strict guidelines. But the DDT era taught the world the consequences of an all-out chemical approach.
The Shift to Biological Control
As DDT and similar chemicals waned, focus shifted to biological and genetic methods to control mosquitoes in safer, more targeted ways:
- Sterile Insect Technique (SIT): Pioneered in the mid-20th century for pests like screwworm flies, SIT involves rearing large numbers of male insects, sterilizing them (traditionally with radiation or chemicals), and releasing them into the wild. The sterile males mate with wild females, but no offspring result, causing the pest population to crash. This technique was adapted for mosquitoes by the late 20th century. While conceptually effective, radiation-sterilized males are often weakened and less competitive in mating, and the approach is labor-intensive (requiring continual mass releases). Nonetheless, SIT demonstrated the power of genetic interventions: you could collapse a pest population via its own mating behavior.
- Biological Control Agents: In parallel, scientists explored using natural enemies or bacterial infections to control mosquitoes. For example, the bacterium Wolbachia can infect mosquitoes and interfere with virus transmission. Releasing Wolbachia-infected mosquitoes is a form of biological (though not gene) control currently used to reduce dengue spread in some countries. This method is non-GMO but is part of the trend toward targeting mosquito biology rather than carpet-bombing with chemicals.
These efforts set the stage for genetic engineering to take mosquito control to the next level.
GM Mosquitoes: The Oxitec Example
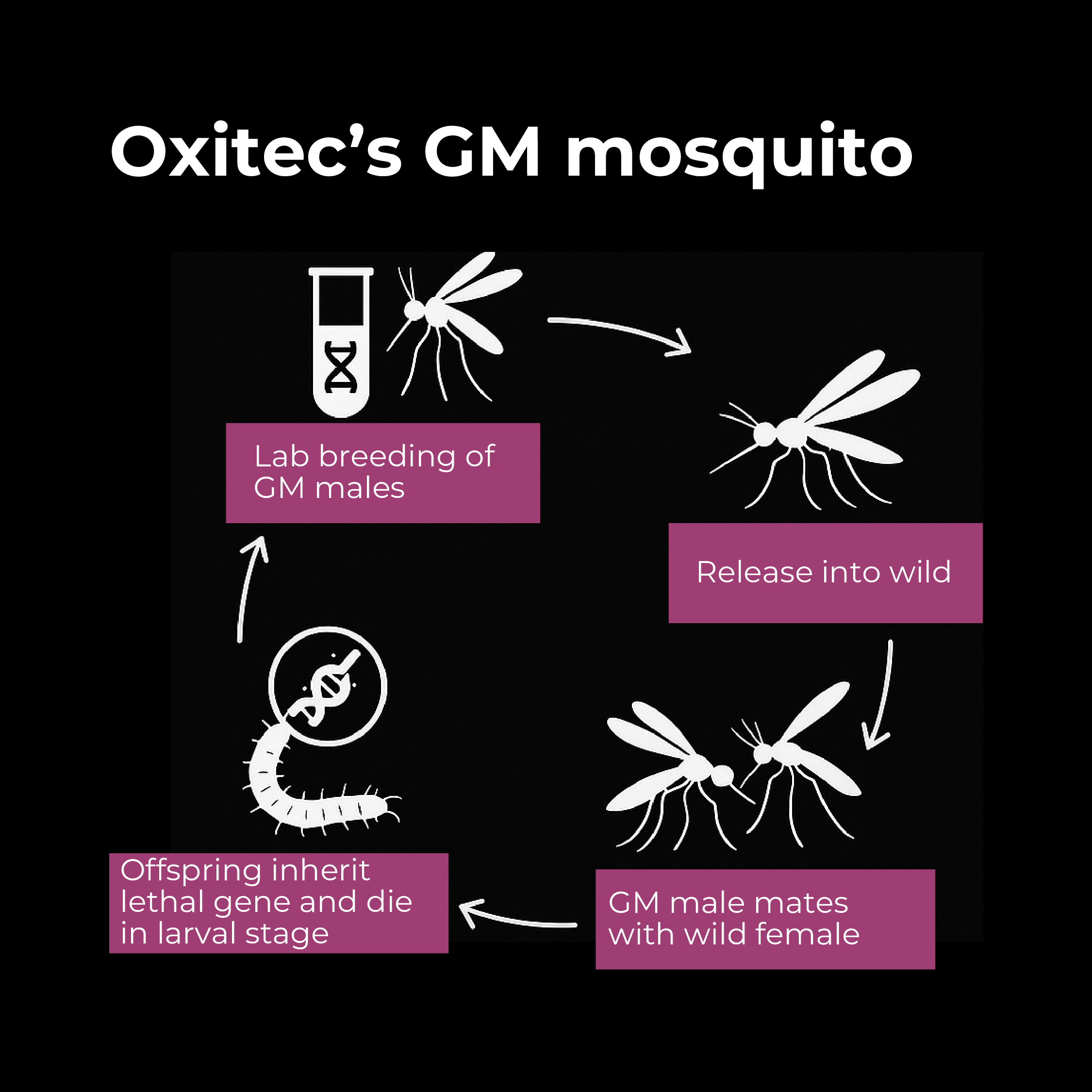
In the 21st century, biotechnology companies began genetically engineering mosquitoes as a novel control method. The British company Oxitec emerged as a leader, developing genetically modified strains of Aedes aegypti (the mosquito species that spreads dengue, Zika, and chikungunya). Here’s how Oxitec’s approach works:
- Lethal Gene Insertion: Oxitec engineered Aedes mosquitoes to carry a “self-limiting” gene. In the lab, they modify male mosquitoes with a gene that produces a lethal protein (often called tTAV) that will kill the insect at a certain life stage. However, the mosquitoes are also given a dietary antidote (tetracycline) in the lab rearing water that allows them to survive to adulthood for release. When these GM males are released into the wild and mate with wild females, their offspring inherit the lethal gene but, lacking the antidote in nature, the larvae die before reaching maturity. In essence, the engineered gene causes any offspring to die, gradually crashing the population.
- Release Programs: Starting in 2009–2010, Oxitec conducted field trials in several countries, including the Cayman Islands, Brazil, Panama, and later Florida in the US. For example, releases in a Brazilian city resulted in up to 95% suppression of the local Aedes aegypti population over time. This dramatic success demonstrated the potential for GM mosquito control to reduce the threat of diseases like dengue fever.
- Regulatory Approval: After years of evaluations, in 2021 the U.S. EPA granted approval for Oxitec to release its GM mosquitoes in pilot programs in Florida and, later, California. The first U.S. trials in the Florida Keys (2021–2022) showed positive outcomes: the modified males successfully mated and passed on the lethal gene, and no female GM mosquitoes (which bite humans) persisted beyond the larval stage. In Brazil, where Oxitec has been active for over a decade, the government has approved large-scale releases. These mosquitoes are often dubbed “friendly mosquitoes” since they are meant to reduce the wild, disease-carrying mosquito population.
Real-world data indicate GM mosquitoes can significantly reduce populations of Aedes aegypti when deployed properly. In neighborhoods where Oxitec conducted releases, the incidence of dengue fever dropped in tandem with mosquito suppression (though robust epidemiological studies are ongoing).
Toward Gene Drives and Future Genetic Tools
Oxitec’s approach is self-limiting – the gene does not spread indefinitely; it just kills the immediate offspring. Next-generation strategies aim for a more permanent solution: gene drives. A gene drive is a genetic mechanism that biases inheritance so that a particular gene is passed on to nearly all offspring, even if it normally would follow Mendelian 50% inheritance. Using tools like CRISPR, scientists have engineered gene drives in labs that force a desired trait through a population with startling efficiency.
In mosquitoes, a proposed gene drive might, for example, spread a gene causing sterility in females, or bias offspring to be all males (which don’t bite or lay eggs). This could crash the population permanently after a single introduction of relatively few engineered mosquitoes, as the drive propagates over many generations until most mosquitoes carry the sterility trait. Researchers in the Target Malaria consortium are working on such drives for Anopheles mosquitoes (malaria vectors).
Future Bio-Threats vs. Bio-Solutions: Gene drives are powerful, but they raise ecological and security questions:
- Benefits: A successful gene drive could potentially eradicate malaria mosquitoes from entire regions, saving hundreds of thousands of lives per year. This is a holy grail for public health. Drives could also target other pests (like eliminating ticks that spread Lyme disease, or invasive rats on islands).
- Risks: Once released, a gene drive might be nearly impossible to retract — it could spread across borders, affecting ecosystems in unforeseen ways. Eliminating a species (even a dangerous one like Anopheles mosquitoes) could have cascading ecological effects, though studies suggest ecosystem impact might be minimal if only one mosquito species is targeted (since many parallel species exist). Unintended mutations in the drive could also occur, potentially creating a strain of mosquito that’s still disease-prone but now harder to control.
- Biosecurity Threats: The same technologies could conceivably be misused to create “weaponized” insects. There have been speculative scenarios of gene drives being used maliciously (for example, to spread a harmful gene in beneficial insects or crops). Also, a rogue actor could engineer mosquitoes to carry a lethal human pathogen or enhance vector competency. While these scenarios belong largely to science fiction at present, experts note that CRISPR and synthetic biology advances are dual-use, meaning they hold great promise for public health but could be misapplied. The world witnessed how pathogens can wreak havoc during the COVID-19 pandemic; an artificially enhanced mosquito-borne pathogen could be a future biosecurity concern, hence oversight is crucial.
In sum, the insect world is becoming a field of genetic battle. We started with broad-spectrum chemicals like DDT, moved to precision genetics like GM and sterile mosquitoes, and now contemplate self-propagating genetic solutions like gene drives. Each step increases the sophistication of our “life chain” interventions: rather than killing mosquitoes directly, we are re-wiring their DNA chains to eliminate themselves. This reduces environmental collateral damage, but as the methods become more powerful, the need for stringent ethical guidelines and safety controls grows. The next section on medical gene editing further illustrates how gene technologies walk a fine line between revolutionary benefits and profound ethical questions.
Medical Revolution: CRISPR and Gene Therapy – Mechanics, Successes, and New Horizons
Advances in genetics have ignited a medical revolution. Tools like CRISPR have made it feasible to edit the human genome, and gene therapy is providing cures for diseases once thought untreatable. In this section, we explain the mechanics of CRISPR and gene therapy, highlight major successes (such as treating sickle cell anemia), and outline other indications where gene editing is making headway.
CRISPR 101: How Gene Editing Works
CRISPR-Cas9 is a groundbreaking gene-editing tool adapted from a bacterial immune system. In simple terms, CRISPR is like molecular scissors guided by a GPS: scientists design a short RNA sequence (the “guide RNA”) that matches a target DNA sequence in a genome, and attach it to the Cas9 enzyme (which acts as scissors). The guide RNA leads Cas9 to the exact desired location on the DNA chain, and Cas9 then cuts both strands of the DNA at that spot. Once the DNA is cut, the cell’s natural repair machinery kicks in. At this stage, scientists can add, remove, or alter genetic material:
- If we want to disable a gene, we let the cell repair the cut in a sloppy way that introduces small errors (mutations) which knock out the gene’s function.
- If we want to insert or correct a gene, we can provide a template DNA sequence. The cell may use this template to repair the cut, thereby incorporating new genetic information.
CRISPR technology, first demonstrated for genome editing in 2012, is faster, cheaper, and far more precise than previous methods of gene editing (like zinc finger nucleases or TALENs). Its ease of use is so striking that it’s often described as making gene editing “democratized” or “as easy as editing text.” Laboratories worldwide swiftly adopted CRISPR for research, and by 2015, the first attempts to use CRISPR to treat diseases were underway.
What is Gene Therapy?
Gene therapy is a broad term for treating or preventing disease by altering a person’s genes. Traditional gene therapy (pioneered in the 1990s) usually involved adding a healthy copy of a gene to cells that have a faulty gene. Early gene therapies used genetically modified viruses (like lentiviruses or adenoviruses) to deliver corrective genes into patient cells. For example, in 1990, a four-year-old girl with SCID (“bubble boy disease”) became the first gene therapy patient – doctors gave her white blood cells a correct copy of the defective ADA gene, partially restoring her immune function.
CRISPR and related genome editors have turbocharged gene therapy by allowing not just addition of genes but editing of the existing genes:
- Ex Vivo Gene Therapy: Cells are taken from a patient, edited in the lab, and then re-infused. This is often done for blood diseases – e.g., bone marrow stem cells can be edited ex vivo and then transplanted back.
- In Vivo Gene Therapy: The editing tool is delivered directly into the patient’s body to modify cells inside the body. This is more challenging (delivery is often via a virus or nanoparticle injection), but successes are emerging for certain liver and eye diseases.
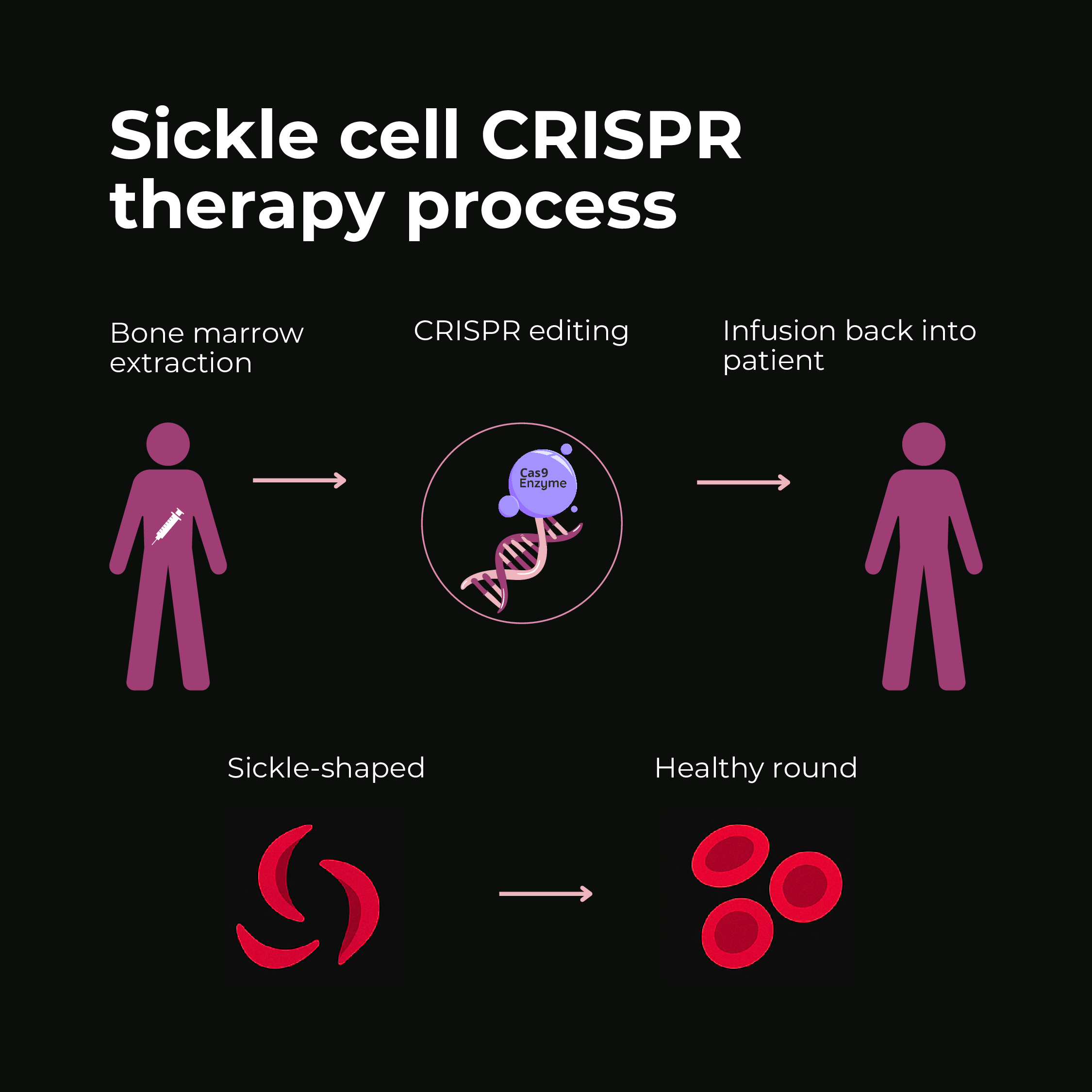
Success Story: Curing Sickle Cell Anemia
One of the most compelling successes of CRISPR-based gene therapy is in sickle cell disease (SCD). SCD is caused by a single mutation in the beta-globin gene, leading to abnormal hemoglobin that makes red blood cells deform into a sickle shape, causing pain and organ damage. For decades, a cure was elusive except for risky bone marrow transplants. Enter gene editing:
- In 2019, a groundbreaking trial treated a patient named Victoria Gray, who became the first person with a genetic disease to receive CRISPR therapy. Doctors harvested her bone marrow stem cells, used CRISPR-Cas9 to edit a gene switch that turns on fetal hemoglobin production (a strategy to compensate for the defective adult hemoglobin), and infused the edited cells back. The result was dramatic – the edited cells produced healthy hemoglobin and freed her from the painful crises of sickle cell.
- As of 2023, dozens of sickle cell (and beta-thalassemia) patients have been treated with similar CRISPR ex vivo therapies. The outcomes have been remarkably positive: Over 90% of treated SCD patients in trials experienced no vaso-occlusive crises (painful episodes) for at least a year after treatment. Many were essentially cured – living without symptoms or transfusions for the first time in their lives.
- This culminated in late 2023 with regulatory approvals. In November 2023, the UK became the first to authorize a CRISPR-based medicine, granting approval to a therapy called Casgevy for sickle cell. In December 2023, the U.S. FDA approved the same CRISPR therapy (developed by Vertex and CRISPR Therapeutics) along with another gene therapy (Bluebird Bio’s Lovo-cel, now called Lyfgenia) for sickle cell. These approvals are historic – Casgevy is the world’s first treatment where patients’ cells are edited by CRISPR and given back as a cure.
Patients like Victoria Gray have described the gene therapy as life-changing. The CRISPR approach for SCD is effectively a functional cure, and it’s a one-time treatment. Unlike chronic therapies, gene therapy permanently fixes or compensates for the genetic defect. As one report noted, “the most groundbreaking thing is that gene therapies only need to be administered once and are essentially curative”, liberating patients from lifelong dependence on medications or transfusions.
Other Indications and Successes
Sickle cell is just one example. The pipeline of gene therapies, many using CRISPR or related technologies, is expanding rapidly:
- Inherited Blood Disorders: Along with sickle cell, beta thalassemia (a severe anemia) has been treated by a similar ex vivo CRISPR strategy. Patients who were transfusion-dependent are now transfusion-free after editing their bone marrow cells to boost fetal hemoglobin. These blood disorders may become the first widely cured genetic diseases via gene editing.
- Blindness (Retinal Diseases): In 2017, the FDA approved Luxturna, a gene therapy (non-CRISPR, using a virus to deliver a good gene) for a rare blindness – making it the first gene therapy for an inherited disease. Building on that, in 2020 researchers used CRISPR inside the human body for the first time to try to treat Leber’s Congenital Amaurosis (LCA10), a genetic retinal disease. They injected a CRISPR therapy (EDIT-101) under patients’ retinas to remove a mutation. Early results showed some modest vision improvement in a few patients, but it is still experimental.
- In Vivo Liver Edits: In 2021, a landmark trial by Intellia Therapeutics treated transthyretin amyloidosis (a deadly protein deposition disease) by infusing patients with lipid nanoparticles carrying CRISPR components. The CRISPR system, delivered to the liver, inactivated the disease gene in liver cells. Within weeks, patients’ blood levels of the toxic protein dropped over 80%. This was the first ever systemic delivery of CRISPR in humans, and it worked with high efficiency. It opens the door for treating many liver-mediated genetic and metabolic diseases via one-time IV infusions of gene editors.
- Cancer Immunotherapy: Gene editing is improving treatments like CAR-T cell therapy. CAR-T involves genetically modifying a patient’s own T-cells to attack cancer. Traditional CAR-T uses viral vectors, but now clinical trials are using CRISPR to create CAR-T cells or other edited immune cells. In one trial, CRISPR was used to remove the PD-1 gene from T-cells (a gene that cancers exploit to disarm T-cells). The edited T-cells were then infused into cancer patients, and initial results showed it was safe and the cells could attack tumors. More broadly, gene-edited immune cells are being explored for solid cancers and to make universal donor CAR-T cells.
- Rare Metabolic and Neurological Diseases: Several rare diseases caused by single gene defects are in the crosshairs. For example, metachromatic leukodystrophy and Hurler syndrome are being targeted by gene therapies delivering correct genes to the brain and body. Duchenne muscular dystrophy, a muscle-wasting disease, saw a gene therapy approved in 2023 (Elevidys) that partially helps by delivering a mini version of the dystrophin gene. Researchers are also attempting CRISPR fixes for Duchenne in animal models. Another promising area is progeria (a premature aging disease) – in 2021, a CRISPR base editor (an enzyme that alters a single DNA letter without cutting) reversed symptoms of progeria in mice, though human use is further out.
- HIV and Other Infectious Diseases: Gene editing is being investigated to make people resistant to HIV by editing the CCR5 gene in T-cells (notably, CCR5 is the same gene altered in the controversial 2018 CRISPR babies, discussed later). Some experimental therapies have tried to knock out CCR5 in bone marrow stem cells and give them to patients, aiming to mimic the natural “CCR5-delta32” mutation that confers HIV immunity. While a functional cure via gene therapy is still in development, the concept is proven by individuals cured of HIV after bone marrow transplants from CCR5-mutant donors. CRISPR might achieve a similar effect without a donor. Additionally, CRISPR-based antivirals are being developed to directly attack viral DNA (like HIV or hepatitis B) within infected cells.
By the end of 2023, the momentum in gene therapy was so great that the FDA stated expectations of approving 10 to 20 gene and cell therapies per year by 2025, given the rich pipeline. Indeed, multiple gene therapies were approved in 2022–2023, including treatments for beta-thalassemia, hemophilia, and a second inherited retinal disorder. The FDA Commissioner in 2019 called this surge a “turning point” comparable to the advent of monoclonal antibodies decades ago.
New Tools: Base Editing and Prime Editing
Beyond CRISPR-Cas9, newer gene editing tools are emerging:
- Base Editors: These are molecular machines that can directly convert one DNA base into another (such as an A to a G or C to a T) without cutting both DNA strands. They are ideal for fixing point mutations. In 2022, a first-in-human trial of a base editor treated a person with T-cell leukemia by editing immune cells to attack the cancer (the trial, called BEACON, is ongoing). Base editing is also being tried in sickle cell (Beam Therapeutics’ BEAM-101 uses a base editor to induce fetal hemoglobin). The advantage is potentially fewer off-target effects and more precise single-letter changes.
- Prime Editing: Introduced in 2019, prime editing is like a “search-and-replace” for DNA. It can perform small insertions, deletions, and all 12 possible point mutations in DNA without making double-strand breaks. Though not yet in the clinic, prime editing could one day correct mutations for genetic conditions that are not easily addressed by CRISPR or base editing.
All these advancements demonstrate that the lexicon of medicine is shifting. Terms like CRISPR, gene therapy, base editing—once obscure technical jargon—are becoming part of mainstream discussions about cures. Publications and governments emphasize that these technologies, while extremely promising, must be used responsibly. As the World Health Organization noted, “human genome editing has the potential to cure disease, but its full impact will only be realized if deployed for all people, instead of fueling inequity”. The next section will delve into the ethical debates and regulatory frameworks ensuring these genetic tools are used wisely and safely.

Ethical Debates and Regulation
The ability to modify the genetic code of living beings—from crops and insects to human patients and even embryos—raises profound ethical questions and regulatory challenges. This section will examine key debates (safety of GM foods, environmental concerns, human gene editing ethics, “playing God” fears) and outline how governments and international bodies regulate genetic technologies to address these issues.
GM Foods and Crops: Safety, Environment, and Consumer Rights
From the start, genetically modified foods have been met with both optimism and skepticism. Key ethical and social issues include:
- Food Safety: Are GM foods safe to eat? Scientific consensus so far is that the GM foods on the market are as safe as conventional foods. These crops undergo rigorous food safety assessments focusing on toxicity, allergenicity, and nutritional effects. For example, the World Health Organization states “GM foods currently available… have passed safety assessments and are not likely to present risks for human health” and notes that no adverse health effects have been detected among the public from approved GM foods. However, watchdog groups sometimes argue that not enough long-term studies exist and point to studies (often disputed or retracted) that claimed potential harm (such as a controversial 2012 rat study by Séralini that linked GM corn to tumors, which was later refuted for poor design).
- Allergenicity and Gene Transfer: Ethical guidelines urge caution about transferring genes from allergenic sources (like peanuts) into other foods. To date, no GM food on the market has been found to cause new allergies in people. Another worry is whether genes from GM foods could transfer to human gut bacteria or cells – e.g., an antibiotic resistance gene used as a marker could, in theory, move into gut microbes. While the probability is extremely low, developers now avoid antibiotic resistance markers when possible.
- Environmental Impact: GMO crops raise ecological questions: Could engineered genes escape into wild relatives (outcrossing) and create “superweeds”? Could plants engineered to produce pesticides (like Bt toxin) harm non-target organisms (like butterflies or beneficial insects)? These are taken seriously in environmental risk assessments. So far:
- Gene Flow: There have been cases of gene flow (e.g., herbicide tolerance from GM canola to wild mustard relatives). Strategies like planting buffer zones and using sterile plant varieties can mitigate this. Regulators assess whether wild plant populations could become invasive if they pick up the trait.
- Non-target Effects: Extensive studies on Monarch butterflies and Bt corn in the early 2000s, for instance, concluded that the risk to monarch populations from Bt pollen was very low under real-world conditions (initial lab studies had raised a concern). Still, monitoring is important. Also, overuse of Bt crops has led to some target pests developing resistance to Bt toxin, which is an evolutionary issue requiring integrated pest management to rotate or stack traits.
- Biodiversity: A common critique is that GM crop monocultures might reduce agricultural biodiversity and promote increased herbicide use (e.g., Roundup Ready crops led to heavy glyphosate use, causing glyphosate-resistant weeds to emerge). On the other hand, some GM traits reduce chemical use (Bt crops need less sprayed insecticide, and herbicide-tolerant crops encouraged a shift to a less toxic herbicide). These trade-offs are part of regulatory evaluations.
- Corporate Control and Farmers’ Rights: Ethical debate often isn’t just about the science: it’s about who controls the food supply. Major GM seeds have been patented by large companies (e.g., Monsanto, now Bayer; DowDuPont/Corteva; Syngenta). Farmers might be legally restricted from saving seeds. This has raised concerns about small farmers’ dependency on big agribusiness and the consolidation of seed markets. Advocates argue for ensuring technology access to the poor (like developing Golden Rice as a humanitarian project, not a profit-driven one).
- Consumer Choice and Labeling: Many argue ethically that consumers have a right to know if their food contains GM ingredients. This led to pushes for GMO labeling laws. The EU has long required labeling of any food with >0.9% GM content. In the United States, after years of debate, a federal law was passed in 2016 requiring bioengineered food disclosure by 2022. However, instead of using the term “GMO”, the U.S. labels say “Bioengineered” and can even be just QR codes on packaging. Critics like food policy expert Marion Nestle argue this was industry-friendly, making the label less obvious (a “bucolic” term and imagery) and excluding many processed ingredients (refined sugars/oils from GM crops are exempt from labeling). The ethical crux is balancing transparency with avoiding undue alarm; GMO proponents worry that a skull-and-crossbones style label would imply a warning not backed by science, whereas consumer advocates insist full transparency is a basic right.
Regulation of GMOs: To navigate these issues, most countries have robust GMO regulatory frameworks. The United States uses the 1986 Coordinated Framework for Biotechnology which assigns roles to the FDA, USDA, and EPA. The FDA evaluates food safety of GM crops (composition, allergens, etc.), EPA regulates any pesticidal substances produced by GM plants (like Bt toxins, termed Plant-Incorporated Protectants), and USDA-APHIS ensures GM plants won’t harm agriculture or become plant pests. Every GMO undergoes years of review before commercialization.
The European Union takes a more stringent approach rooted in the precautionary principle. Approvals at the EU level have been slow and contentious, and member states can opt-out of cultivation. Europe’s skepticism is partly cultural and partly due to past food safety scandals (like mad cow disease) that eroded public trust. As of 2023, the EU is actually re-evaluating its stance for new gene-edited crops, considering easing rules for certain “new genomic techniques” due to pressure to innovate and adapt to climate change. The debate pits potential agri-tech benefits against deep-seated public caution.
On the international stage, the Cartagena Protocol on Biosafety (2003) provides a framework for cross-border movement of GMOs. It allows countries to ban imports of GMOs if there’s insufficient evidence of safety, reflecting the precautionary stance. It also mandates labeling shipments of GM commodities like corn or soy. Over 170 countries have ratified this treaty (notably, the US has signed but not ratified). Cartagena embodies the ethical priority of “do no harm” across national boundaries and the right of nations to protect biodiversity from possible risks of foreign GMOs.
Human Gene Editing: Ethics of CRISPR and the Germline
The ethical stakes become especially pronounced with human gene editing, particularly editing that can affect future generations (germline editing):
- Somatic vs. Germline: Somatic gene therapy (editing cells that are not passed to offspring, like blood or organ cells) is relatively uncontroversial as a medical treatment, as it affects only the treated individual. Germline editing (changing embryos, sperm, or eggs) would be heritable, passing changes to descendants. This raises far deeper ethical questions because it could permanently alter the human gene pool.
- CRISPR Babies Controversy: In November 2018, Chinese scientist He Jiankui shocked the world by announcing the birth of the first gene-edited babies. He had used CRISPR on human embryos to disable the CCR5 gene (in an attempt to make the babies HIV-resistant, as their father was HIV-positive). This was done in secrecy and implanted into a woman, resulting in twin girls. The scientific community and ethicists were nearly unanimous in condemnation. Why?
- The experiment was premature and medically unnecessary (HIV can be prevented by safer means; editing embryos for this was unjustified risk).
- He Jiankui conducted it without proper oversight or transparency, violating norms and possibly Chinese regulations on embryo research.
- The edits themselves were off-target and not the exact intended change (the twins’ CCR5 genes were mosaics, not the known Delta32 mutation that gives HIV resistance). This means the experiment failed on technical grounds too, yet still resulted in living children with unknown mutations.
- Importantly, any mistakes could potentially harm not just these children but their future offspring. Informed consent was also dubious—embryos can’t consent, and the parents may not have been fully aware of risks (reports later showed He obscured details in consent forms, describing it misleadingly as an “AIDS vaccine” project).
The affair led to Dr. He’s swift punishment: in December 2019, a Chinese court sentenced him to 3 years in prison and a fine for illegal medical practice. Two colleagues were also jailed for shorter terms. The clear message: human germline editing in embryos is off-limits – at least for now – and doing it in secret is criminal. Worldwide, this event prompted deeper discussions and calls for stronger governance of gene editing.
- Ethical Concerns in Human Editing: Scholars often frame them around principles:
- Safety: Is it safe and free from off-target effects or mosaicism? With current tech, germline editing is not safe enough to consider in embryos destined to become babies. Even somatic therapies must be cautious of unintended edits.
- Consent: Future generations cannot consent to the genetic changes we may impose on them. Some argue parents routinely make choices affecting their kids (like IVF with screening), but germline changes are qualitatively different.
- Justice and Access: If germline editing were allowed, would it be accessible or only for the rich? There are fears of exacerbating inequality—e.g., designer babies with superior traits for those who can pay, versus a natural underclass. The WHO’s Director-General Tedros warned that we must ensure genome editing doesn’t increase health inequity.
- Line between Therapy and Enhancement: Most ethicists support gene editing for serious diseases (once safe) but strongly oppose it for enhancements (like choosing eye color, height, intelligence). However, the boundary isn’t always clear-cut. Is reducing Alzheimer’s risk an “enhancement” or prevention? The slippery slope worry is real: once germline editing is normalized for disease, what stops a drift toward non-medical enhancements? Many find germline editing for anything other than dire medical need morally problematic, at least until society builds consensus.
- Human Dignity/Natural Integrity: Some argue that making designer humans, even with good intent, could undermine the appreciation of human life as a gift. Others see it as the next step in alleviating suffering. This touches philosophical or religious beliefs about “playing God” or altering “what it means to be human.”
- Global Policy: Around 40 countries explicitly ban or restrict human germline editing in law (e.g., many in Europe). Others have guidelines but perhaps not hard laws. The 2015 International Summit on Human Gene Editing in Washington concluded it’d be irresponsible to proceed with any clinical germline editing until safety issues are resolved and there’s broad societal consensus. After the CRISPR babies scandal, leading scientists called for a global moratorium on implanting gene-edited embryos (March 2019, published in Nature). In 2020 and 2021, the WHO convened an expert panel, which in mid-2021 recommended a global registry of gene editing research and advised the world to stick to somatic editing for now, and keep germline editing banned “until conditions for acceptability are met”. They emphasized international governance to prevent “illegal, unregistered, unethical or unsafe research”. The fact that He Jiankui could do what he did indicated gaps in oversight.
- Recent Developments: Interestingly, in 2023, after serving his sentence, He Jiankui re-emerged and expressed interest in working on gene therapy for diseases—he was met with wariness; the global scientific community hasn’t fully forgiven the breach of trust. Meanwhile, other scientists like the Russian Denis Rebrikov at one point voiced plans to edit embryos to fix a deafness gene (he did not proceed amid pushback). The bottom line: there is a de facto international consensus that clinical germline editing is on hold, but research on it (in labs, not resulting in births) continues in order to improve safety, in case society decides to use it in the future for serious diseases.
Regulation in Medicine and Gene Therapy
Regulating medical gene editing and therapies is complex but critical:
- Clinical Trials Oversight: In countries like the U.S., somatic gene therapy is regulated as a biologic drug by the FDA, which requires years of clinical trials. Ethics review boards and institutional protocols ensure that any experiment (like CRISPR trials) have informed consent and scientific merit. The tragic death of a patient (Jesse Gelsinger) in a 1999 gene therapy trial due to immune reaction led to more stringent monitoring and transparency in gene therapy studies.
- Post-market Monitoring: Even after approval, gene therapies are often subject to long-term follow-up studies, since modifications could have lasting effects. For instance, some early gene therapy trials saw leukemia as a side effect due to where the new gene inserted itself. Regulators now often require integration site analysis and decades-long patient registries for gene therapy recipients.
- CRISPR Patent and Access Issues: There has been a major patent dispute over CRISPR (groups from Broad Institute vs UC Berkeley). This affects which companies can develop therapies and can impact cost due to licensing fees. On the access side, gene therapies are extremely expensive. The cost of the one-time cures like Zolgensma for SMA is $2.1 million, making it the world’s most expensive drug. Ethical questions arise: How can healthcare systems afford these? Is it ethical that a cure exists but might be denied to patients due to cost? Companies argue the prices reflect long-term value (Zolgensma takes the place of many years of medical care), but many call for new models (outcomes-based payments, or govt/charity subsidies) so that “no patient is left behind” by these breakthroughs.
- Biosecurity and Dual-Use Regulation: As mentioned, CRISPR could be misused to create bioweapons. Authorities are looking at strengthening the Biological Weapons Convention to address genome editing. There are also discussions about screening DNA synthesis orders (companies that make custom DNA for researchers screen sequences to prevent someone printing a dangerous virus genome, for instance). The challenge is to allow open research and innovation while guarding against misuse – a classic dual-use dilemma.
Societal Discourse and Future Guidelines
Ethical debates extend into society at large. Public engagement and education are key. In 2018-2019, many science bodies, like the U.S. National Academies and the UK’s Royal Society, held international commissions on heritable human genome editing. Their 2020 report outlined a cautious roadmap: it did not green-light clinical use, but said if someday society considers it, it should be limited to serious diseases with no alternatives, and only after much more research and broad consensus.
For agriculture, some suggest a middle ground: focus on GMO traits that deliver consumer or environmental benefits (e.g., nutrient-enhanced crops, or crops that reduce need for fertilizer) to gain public trust, rather than primarily traits that benefit only producers (like herbicide tolerance). Next-gen gene edited crops (which often don’t contain foreign DNA) might be more palatable and are already being regulated more leniently in countries like the U.S., Japan, Brazil, and potentially soon in the EU. Transparent communication and independent public-interest research (e.g., on long-term ecological impacts) can help address concerns.
In summary, ethics and regulation act as the connective tissue in the chain from genetic science to society. They strive to maximize benefits (disease cures, food security) while minimizing harms (health risks, ecological disruption, inequality). This balance is delicate. The world is essentially conducting a grand dialogue on what is acceptable: Should we alter species? Should we alter ourselves? If so, how far and who decides? We have international treaties like Cartagena for GMOs and the Oviedo Convention (in Europe) against germline editing, but technology is fast outpacing policy. As we move to the conclusion, we’ll consider where this genetic revolution might lead us in the next decade, assuming ethical guardrails guide it responsibly.
Conclusion: What Genetic World Awaits Us in 10 Years?
The pace of advancement in genetics suggests that the world of 10 years from now (circa 2035) could be markedly different. We are, today, at an inflection point analogous to the dawn of the computer or internet age—except this time it’s the code of life we are mastering. Barring unforeseen setbacks, here are some plausible developments and scenarios in the coming decade:
Medicine Transformed
In a decade, numerous genetic therapies may move from experimental to routine:
- Mainstream Gene Therapies: By 2035, it’s expected that dozens of gene therapies will be approved for a range of rare diseases – from muscular dystrophies to metabolic disorders. What’s rare today (only a few thousand patients worldwide) collectively affects millions when added up. Each year, new “one-time cures” should roll out. Regulators predicted that by 2025 onward, 10-20 cell/gene therapies per year could gain approval, so by 2035, there could be a catalog of 100+ gene therapies. This may include CRISPR-based therapies for common conditions like high cholesterol or chronic infections (researchers are already trialing a CRISPR cure for HIV in vivo).
- Cancer and Immune Boosts: Many cancers, especially blood cancers, will likely be treated with genetically engineered immune cells as a standard of care. CAR-T therapies will improve (tackling solid tumors too) and might be gene-edited to reduce side effects and cost. We may even see preventative genetic vaccines – e.g., editing immune cells in healthy but high-risk individuals to pre-arm them against certain cancers.
- CRISPR in the Clinic: CRISPR-based in vivo injections could treat prevalent diseases. For instance, a single IV infusion of a CRISPR therapy might permanently lower a person’s LDL cholesterol by editing the PCSK9 gene in their liver, slashing heart disease risk (human trials are starting). Or CRISPR might be used to remove hepatitis B virus from infected liver cells. These would have global health impact on millions, not just rare cases.
- Cures for Blood Disorders: Sickle cell and thalassemia are likely to be largely cured conditions in many countries, as ex vivo CRISPR treatments get scaled up. The challenge by 2035 will be making these cures accessible in Africa and South Asia where they’re most needed – perhaps simpler in vivo gene editing (injectable) will emerge to avoid bone marrow transplants.
- Accessible Sequencing and Personalized Medicine: By 2035, it may be routine to sequence newborns’ genomes at birth (with parental consent) to identify genetic risk factors or diseases early. This can inform personalized preventative care or early interventions (including gene therapies). Genetic screening before having children might also become more commonplace as part of family planning, raising its own subtle ethical issues but potentially reducing severe genetic conditions via informed choices (IVF with embryo screening, etc.).
- Xenotransplantation: This is tangential but related – genetically modified pig organs for human transplant. In 2022, a man lived two months with a GM pig heart (the pig had 10 gene edits to reduce rejection). In 10 years, such transplants might last much longer, perhaps becoming a bridge to save patients until a human organ is available, or even as semi-permanent solutions. If successful, no one might die on an organ transplant waiting list by 2035 because pig organs can fill the gap, courtesy of genetic engineering.
- Cost and Equity Efforts: With more gene therapies, there will be intense efforts to lower costs: maybe manufacturing innovations, or wider adoption leading to economies of scale. Also, global partnerships may bring therapies to low-income regions at cheaper prices (similar to HIV drugs scale-up in the 2000s). Ethically, the narrative of the next decade will be ensuring these miracle cures do not become a luxury only for rich nations. The WHO and others are actively working on frameworks for gene therapy access as part of universal health coverage goals.
Our Food and Planet
Agriculture and environmental management in 10 years will likely be heavily influenced by genetics:
- Gene-Edited Crops Everywhere: The 2020s’ gene editing boom will yield a suite of new crop varieties by 2030s. Expect drought-tolerant, saline-resistant crops to combat climate change’s effects, possibly achieved by tweaking native genes (no transgene). These could help stabilize yields as weather becomes more erratic. There may also be nutrient-enhanced crops (e.g., biofortified cassava or sorghum) developed via CRISPR that improve food nutrition in developing regions.
- Sustainable Farming: We could see crops engineered to fix nitrogen or use fertilizer more efficiently to reduce runoff pollution. Similarly, gene drives might be applied to pests to protect crops without chemical pesticides. For example, a gene drive could curb locust swarms or beetles that devastate crops. By 2035, if regulatory hurdles are overcome, a few agricultural gene drives might be field-tested.
- Livestock and Aquaculture: There may be gene-edited cows that resist certain diseases (like a TB-resistant cow), or pigs that are immune to swine fever—some of these are in development now. Edited farm animals could improve animal welfare too (e.g., hornless cattle created by knocking out the horn gene, sparing them painful dehorning). In fish farming, more GM fish (beyond salmon) might appear to meet protein demands with smaller environmental footprints.
- Ecological Restoration: Genetics could aid conservation – for example, reviving genetic diversity in endangered species by editing out deleterious mutations or even attempting de-extinction (resurrecting extinct species’ traits in closely related species). While true de-extinction (like a woolly mammoth) is ambitious and controversial, genetic tools may help existing species adapt. By 10 years, we might have, say, some American chestnut trees that are blight-resistant thanks to a gene edit, allowing a near-extinct tree to flourish again, or coral reefs revived by gene-tweaked corals that withstand warmer oceans.
- GMOs and Society: Public acceptance might shift as people directly experience benefits. If, for instance, a CRISPR-engineered rice strain significantly reduces childhood blindness (as Golden Rice aims to do by providing vitamin A), it could soften opposition. Education will play a role; the next generation, growing up amid daily news of CRISPR breakthroughs, may view genetic tech as normal tools rather than uncanny science. This could lead to more balanced public dialogues and nuanced regulations that differentiate between types of modifications.
Genetic Insect Control and Public Health
The coming years may well bring the first deployments of advanced genetic biocontrol:
- Malaria Mosquito Gene Drive: A significant possibility is that by the early 2030s, if all safety tests go well and regulatory pathways are established, a gene drive mosquito could be released in Africa to fight malaria. Organizations like Target Malaria are aiming for field use perhaps within ~5-10 years. Success could mean the permanent elimination of certain malaria-transmitting mosquito populations, potentially eradicating malaria in some regions. This would be a world-changing event, though it will be approached with extreme caution and rigorous monitoring.
- Other GM Insects: Following mosquitoes, other disease vectors might be tackled. Dengue-spreading Aedes mosquitoes via gene drives, or ticks edited to reduce Lyme disease spread, might be on the horizon. Also, beneficial uses like genetically boosting honeybee resistance to viruses (to combat colony collapse) could be seen.
- Bio-threat Preparedness: On the flip side, as gene tech proliferates, the world will have to invest in biosecurity measures. By 2035, we may have international agreements explicitly covering gene editing under the Biological Weapons Convention, and improved detection systems for any genetically novel organisms introduced in the environment. The hope is that responsible governance and good actor use of gene tech vastly overshadow any nefarious uses.
Human Germline: Cautious Steps?
In the conservative view, heritable human genome editing will remain banned or on hold through 2035. The focus will be on perfecting somatic therapies and establishing consensus. However, there is a scenario where controlled, narrow use might begin to happen:
- If CRISPR and next-gen tools become extremely precise and safe, and if a compelling medical case arises (say a couple who both have a severe genetic disorder and every child would inherit it – something not avoidable by embryo screening alone), there could be pressure to allow editing embryos to avoid tragedy. By late 2020s or 2030s, regulators might consider specific exemptions for germline editing for disease prevention, under strict oversight, once society has deliberated. But this will be incremental. Countries like the UK (with its robust IVF regulation authority) might lead in allowing limited cases (similar to how they allowed mitochondrial replacement “3-parent babies” for mitochondrial diseases). Any such step would be accompanied by global guidelines to prevent a rogue fertility clinic “free-for-all.” WHO’s 2021 framework is already a step toward such global governance.
- Ethical discourse will continue to be vibrant. We might see publics become more comfortable with some enhancements (e.g., editing out a Alzheimer’s risk gene might be seen as akin to a vaccine). Alternatively, societies could reaffirm a hard line that germline edits are off-limits. Likely, within 10 years, no country will openly permit “designer babies” for non-medical traits, and any transgressions would be met with harsh penalties—learning from the He Jiankui incident.
Democratisation and Literacy
The next decade should also bring greater genetic literacy among the general public. Genetics will pervade education (schools teaching CRISPR as basic knowledge, maybe even hands-on CRISPR labs in high school biology). Citizen science in genetics might bloom, with enthusiasts performing simple gene edits in bacteria or plants in community labs — though regulated to ensure safety. This democratization can spur innovation but must be balanced with regulation to prevent DIY bio misadventures.
Final Thoughts
In the “genetic world” of 10 years hence, humans will increasingly act as stewards of evolution. We will be able to shape crops, creatures, and possibly our own biology with intention and intelligence. The chains of DNA, once understood, have become chains we can link or unlink. This holds tremendous promise: elimination of genetic diseases, more sustainable agriculture, protection of species, and improved healthspan. But with power comes responsibility. Society will need strong chains of ethics and policy to guide these capabilities—“life chains” of accountability that ensure we do not compromise our environment or humanity in pursuit of progress.
In essence, we are writing the next chapter of life’s code. The year 2035 will likely mark the end of the first draft of that chapter, showing us which narratives—utopian or cautionary—gain momentum. Will we see a world free of malaria, with cured patients of sickle cell and cancer, and food security improved by resilient crops? Quite possibly yes12. Simultaneously, we must strive to make this genetic renaissance inclusive and safe: global oversight, equitable access, and public engagement are key. If we succeed, the genetic revolution will be remembered as one of humankind’s greatest triumphs, a time when knowledge of DNA’s double-helical chain empowered us to forge a healthier, more sustainable, and more equitable “life chain” for future generations.

